Introduction
The Integrated National Health System (INHS) allows the worker or retiree and their families to have universal health coverage through a contribution proportional to their income, with the option of a public or private healthcare provider. This determines universal and equitable care coverage for the entire population. In 2004, with the arrival of the first high-cost drugs, rituximab and imatinib, healthcare providers had difficulties financially covering them.
The National Resource Fund is a non-state public Entity created in 1980 to provide coverage for highly specialized medical techniques. It currently provides universal financial coverage to all residents linked to the INHS and encompasses almost all the coverage of patients nationwide. On January 1, 2005, financial coverage for high-cost drugs began with rituximab and imatinib under regulations that required strict diagnosis, treatment, and follow-up criteria.
Non Hodgkin Lymphoma incidence in Uruguay is 11,53 cases per 100,000 men/year, and 7,9 cases per 100.000 women/year. Uruguay's population is estimated in 3,400,000.
This study shows the results obtained with rituximab in DLBCL.
Methods
Analytical retrospective study of a historical cohort of consecutive patients with DLBCL who started first line treatment with chemo-immunotherapy with rituximab between January 1, 2005 and December 31, 2019. Follow-up was conducted until March 8, 2021.
All patients signed informed consent.
Results
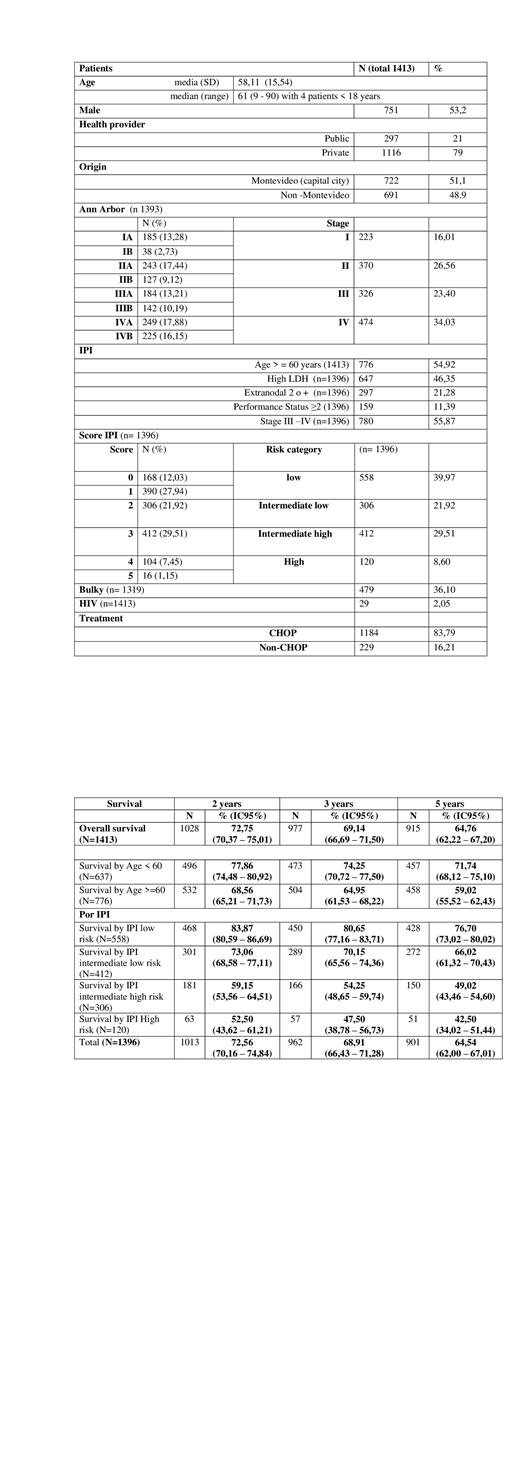
In the period considered, 1699 requests for rituximab treatment for patients with DLBCL were received, 1597 (94%) treatments were authorized. The results of the 1413 (89%) patients with onset of the disease are analyzed, 51,1% were from the capital city, Montevideo and 48,9% from other cities, 21% from public hospitals and 79% from private health system.
Median age was 61 years (9-90), 4 patients < 18 years. Male patients 751 (53.2%)
Regarding the characteristics of the disease, Ann Arbor stage was I in 223 (16.01%), II in 370 (26.56%), III in 326 (23.40%) and IV in 474 (34.03%) of the patients. IPI Score from 0 to 5 were 168 (12.03%), 390 (27.94%), 306 (21.92%), 412 (29.51%), 104 (7.45%), 16 (1.15%) respectively. The most frequent components of the IPI score were age >=60 (54.92%), elevated LDH (46.35%), and Ann Arbor stage III-IV (55.87%). B symptoms were present in 38.19% of the patients and Bulky disease in 479 patients (36%).
HIV diagnosis was recorded in 2.05% of patients (n=29).
CHOP with Rituximab was the treatment in 83.79% of the patients.
With a median follow-up of 84.8 months, the median overall survival (OS) for the study population was 126.9 months (95% CI 102.6 - 138.3). The OS rate at 2 years was 72.75% (95%CI 70.37 - 75.01), at 3 years 69.14% (95%CI 66.69 - 71.50) and at 5 years 64.76% (95%CI 62.22 - 67.20)
In the IPI univariate analysis, age > 60, Ann Arbor Stage III-IV, elevated LDH, and PS>=2 were associated with shorter survival time, but extra-nodal involvement greater than or equal to 2 was not. These prognostic associations were maintained when performing a multivariate analysis.
Median OS according to the IPI for low-, low-intermediate, high-intermediate, and high-risk patients was 190.8, 138.2, 45.5, and 27.9 months, respectively. (p=0.00001).
The 2 years OS for patients < 60 years was 77.86% (95% CI 74.48 - 80.92) while in those > 60 years was 68.56% (95% CI 65.21 - 71.73) (P<0.00001).
Median OS was 114 months and 129 months for patients from public and private health system respectively (p=0.7)
Discussion
The results of OS and its relationship with the factors that make up the IPI are those expected according to the studies that determined that the use of rituximab associated with CHOP was the standard in DLBCL.
Access to the medication was universal for the entire population of Uruguay, we found a lower-than-expected number of patients from the public health system.
Conclusions
The regulations provided the possibility of universal use of rituximab in DLBCL; the diagnosis, treatment and follow-up of these patients was standardized throughout the country. This is reflected in results comparable with international standards in the public and private health system. The survival results found in this study are those expected according to the reference works used in the design of the rituximab coverage regulations.
Said regulation was the beginning of financial coverage by the National Resources Fund for other high-cost medicines.
Disclosures
Muxi:Janssen: Other: Myeloma meeting travel financing; Nolver: Honoraria.